









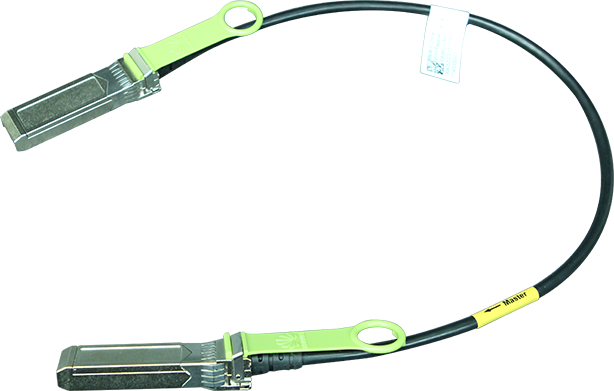







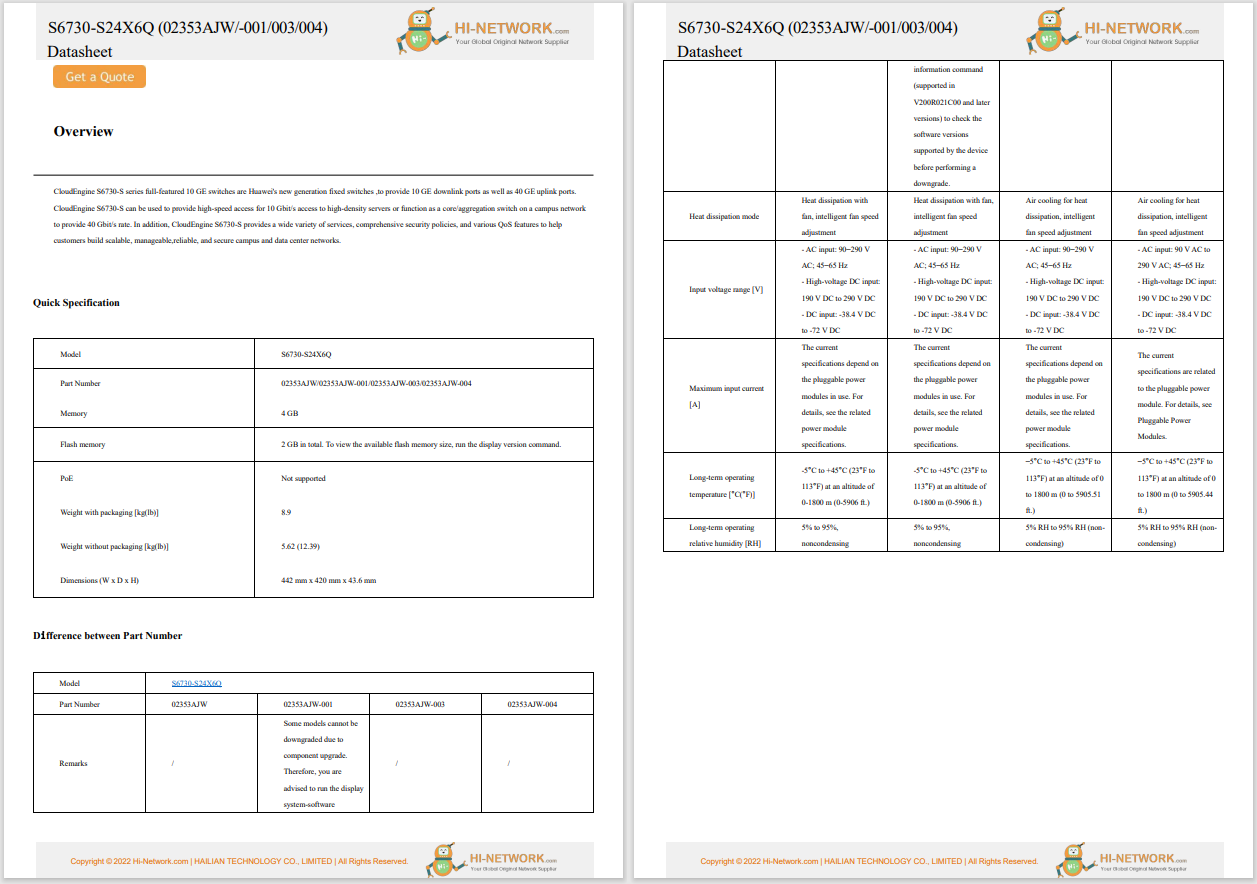
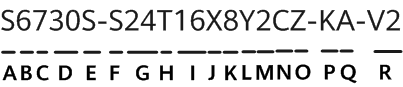













The US Food and Drug Administration (FDA) published an update on digital health software precertification (pre-cert) programme. The pre-cert programme was launched in 2019 to address the challenge that the FDA's traditional approach to regulate hardware based medical devices that are not well suited for faster and more iterative design, development, and validation techniques applied to develop high quality, safe, and effective software, including software as a medical devices (SaMD). The update unpacks learnings to date from the building and testing of the pre-cert pilot programme. It also underscores that the COVID-19 pandemic further highlights the importance of enabling rapid access to safe and effective devices for public health.
 Tags quentes :
Saúde digital: aplicações tecnológicas
Tags quentes :
Saúde digital: aplicações tecnológicas
Cadastre-se agora para Ações Semanais de Promoção
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel em HK: 00852 66181601
Email: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português