









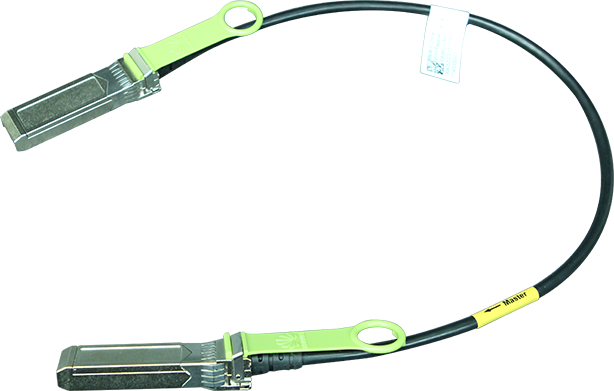




















The US Food and Drug Administration (FDA) granted 23andMe a 510(k) clearance that allows the company to offer a pharmacogenetics report on two medications: clopidogrel (prescribed for certain heart conditions) and citalopram (a depression medication). With the new clearance, 23andMe can provide interpretive drug information to customers for two medications without a confirmatory testing. 23andMe Chief Legal and Regulatory Officer Kathy Hibbs said that the clearance is a testament to the clinical validity of 23andMe results. In the decision, the FDA stated that the 23andMe pharmacogenetic report is a safe and effective consumer product that can support customers with certain genotypes to understand how their body may respond to certain medications and encourage conversations with their healthcare provider.
 Tags quentes :
Saúde digital: aplicações tecnológicas
Tags quentes :
Saúde digital: aplicações tecnológicas
Cadastre-se agora para Ações Semanais de Promoção
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel em HK: 00852 66181601
Email: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português