




























 StackCommerce
StackCommerce The numbers are alarming.
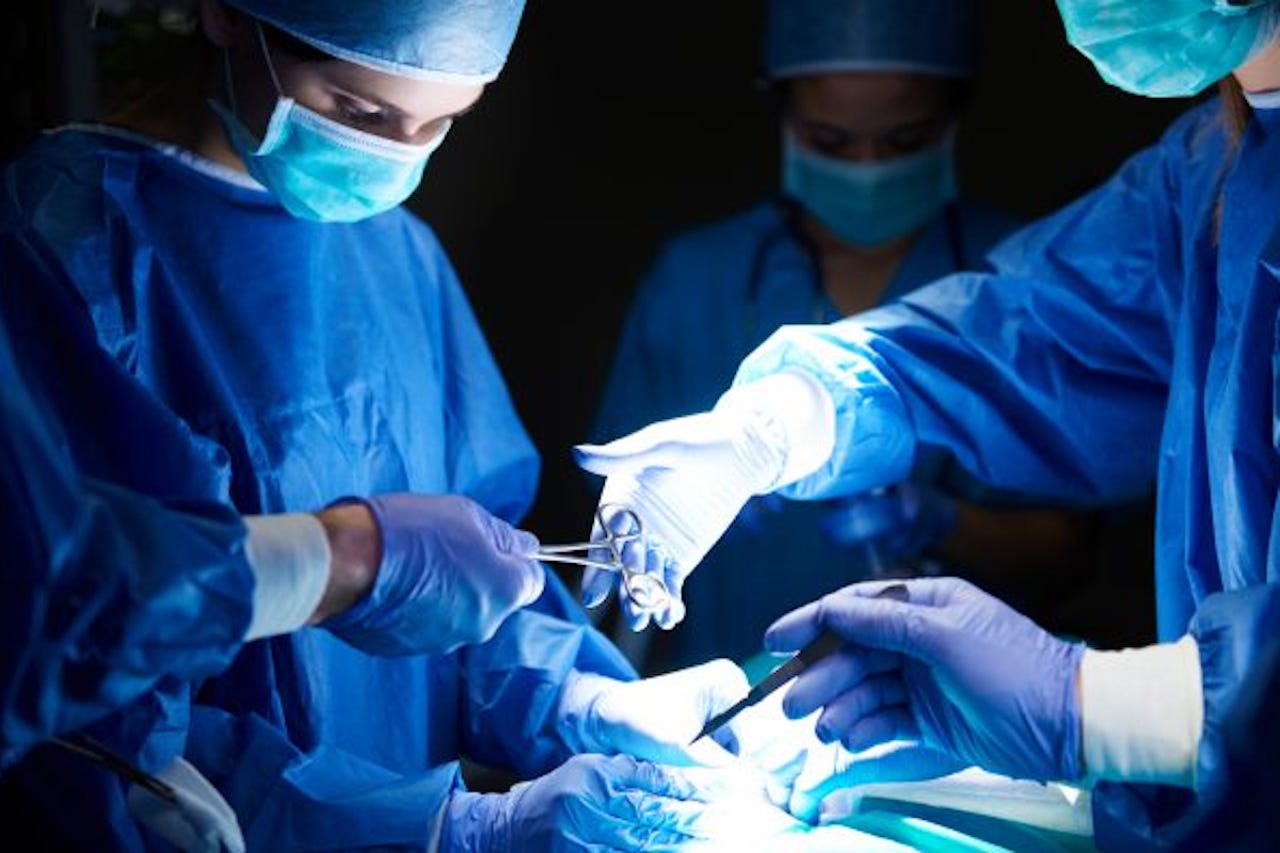
In almost 300,000 cases each year, U.S. surgical patients develop a harmful or potentially deadly infection linked to their procedure. Those infections can happen in virtually any hospital under any doctor's care, drastically altering a patient's recovery. On average, a surgical site infection (SSI) can extend a hospital stay by up to 10 days and increase the chances of having to be readmitted to the hospital later by up to four times. Most concerning, SSIs and resulting sepsis combine to kill 270,000 Americans every year.
And then, there's the money. In all, postoperative infections land a crippling blow to the bottom lines of both healthcare providers and patients alike, causing nearly$34 billion in added costs across the U.S. healthcare system per year. Thankfully, Covira Surgical has launched a novel approach to lowering the rate of postoperative infections.
All it takes is a simple drink.
Currently, most post-op infections are treated with popular bacteria-killing antibiotics. However, a growing number of harmful pathogens have started developing resistances to antibiotic medications. On top of that, antibiotics themselves can cause further damage to a healing body.
Covira Surgical's formulation Pi-PEG doesn't use antibiotics. Instead, this colorless, tasteless solution that a patient drinks before and after surgery focuses on building up the body -- rather than destroying bacteria. When ingested, Pi-PEG prevents the spread of infection by bolstering the gut microbiome, the natural balance in a body's ecosystem for stabilizing and repairing injuries or illnesses.
By stimulating the production of phosphate, Pi-PEG creates an environment that quiets invasive bacteria. It doesn't kill it, but it does greatly suppress its virulence, stopping the growth of infections while strengthening the gut health to further keep any harmful pathogens in check during surgical healing.
So far, Pi-PEG is seeing all green lights during its testing period. This brainchild, the result of 30 years of study by noted postoperative infection specialist Dr. John Alverdy, has already proven effective through animal testing. Now, the company has embarked on the 14-month process of earning U.S. Food and Drug Administration approval for the start of human testing.
Assuming it clears those hurdles, Covira Surgical stands poised to have a new anti-infection treatment available to hospital and surgical providers everywhere, all at a cost estimated at about$200 per patient. That could prove to be a game-changing shift in how post-op treatment is administered, leading to projections of nearly$2.2 billion in revenue for Covira over the next 15 years.
As testing continues, Covira Surgical has also opened up a new crowdfunding campaign, allowing interested investors to buy into the biotech firm for as low as$250. Potential investors can head to this StartEngine page, read the business plan, and decide whether to join the hundreds of others who have invested in the Chicago-based company.
ZDNet may receive monetary compensation by the issuer, or its agency, for publicizing the offering of the issuer's securities. ZDNet and the issuer of this offering make no promises, representations, warranties, or guarantees that any of the services will result in a profit or will not result in a loss.
 Tags quentes :
Nosso processo
Mais Tópicos
ZDNET recomenda
Tags quentes :
Nosso processo
Mais Tópicos
ZDNET recomenda