















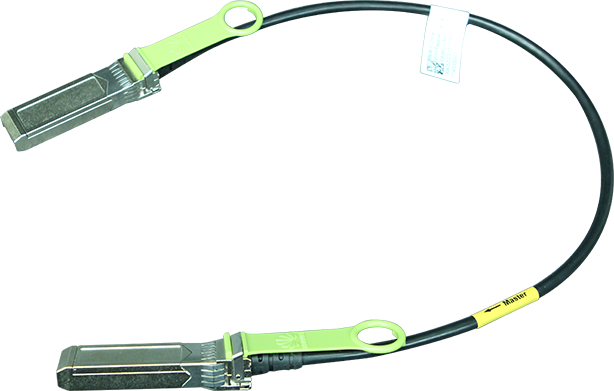







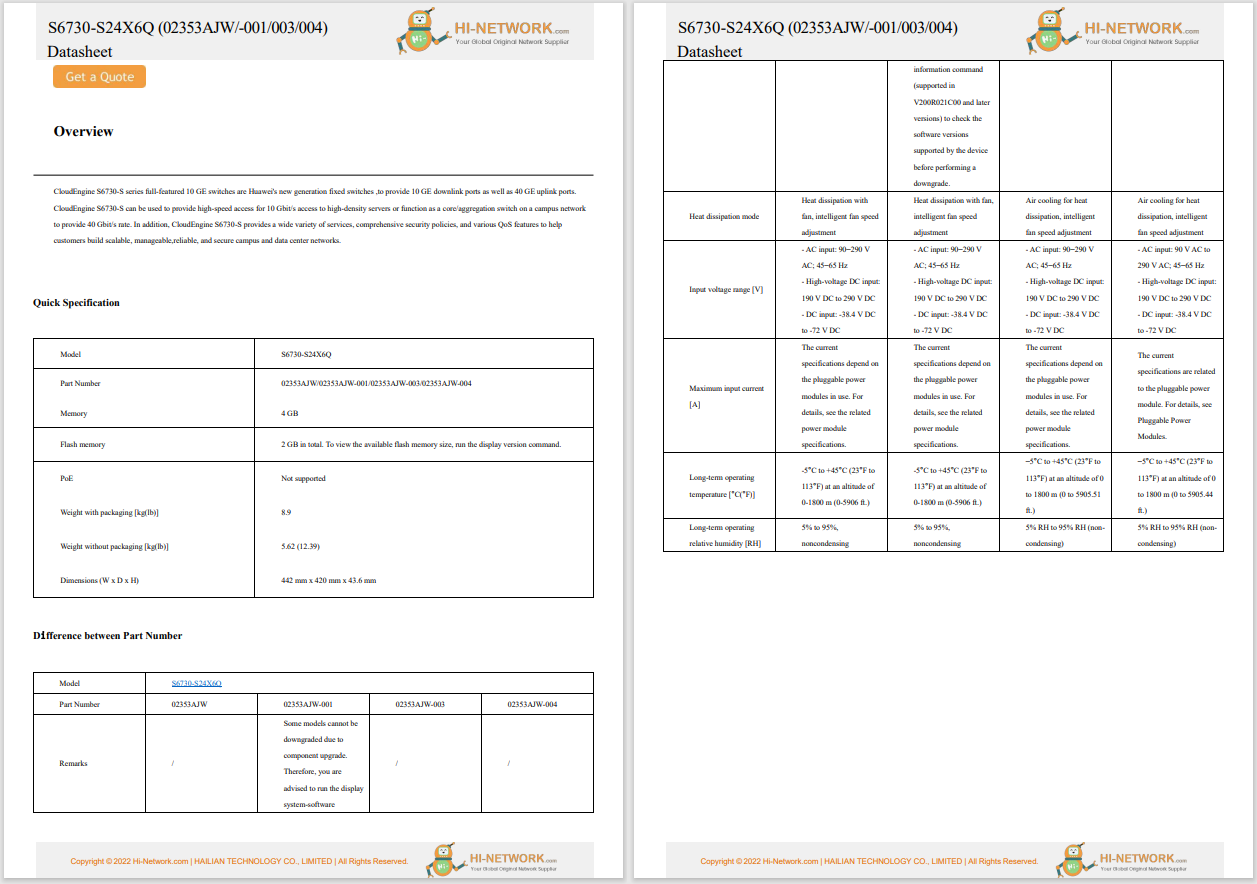
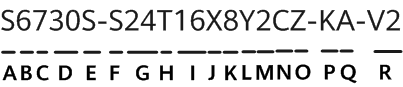







Specialists at the Oregon Health & Science University in Portland (USA) and pharmaceutical companies Editas Medicine of Cambridge, Massachusetts (USA), and Allergan of Dublin (Ireland) are conducting a clinical trial, called Brilliance. During the clinical trial, CRISPR-Cas9 gene therapy is administered directly into the body of a person with a genetic condition that causes blindness. The components of the gene editing system are encoded in the genome of a virus and then injected directly into the eye, with the purpose of deleting a mutation in the gene responsible for the patient's condition. This is the first time when the CRISPR-Cas9 technique is deployed directly into the human body; previous trials have seen CRISPR-Cas9 used to edit cell genomes removed from the body and later infused back.
 Tags quentes :
Tecnologias emergentes
Tags quentes :
Tecnologias emergentes
Cadastre-se agora para Ações Semanais de Promoção
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel em HK: 00852 66181601
Email: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português