









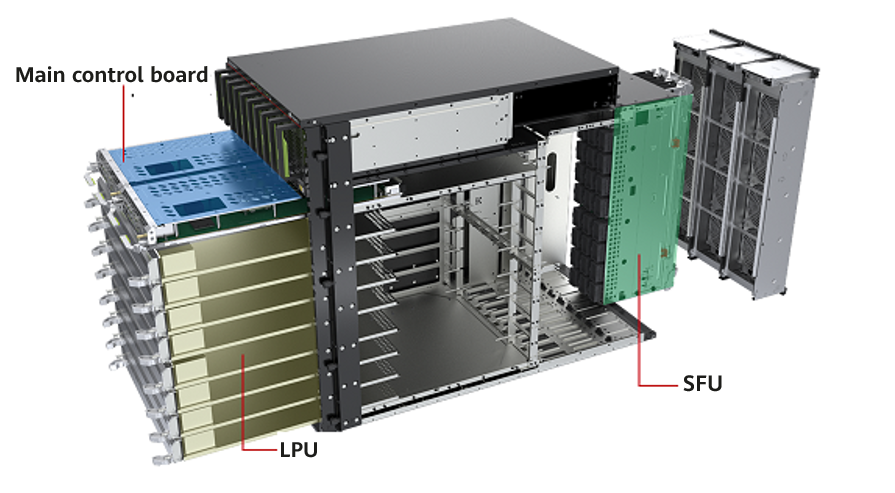

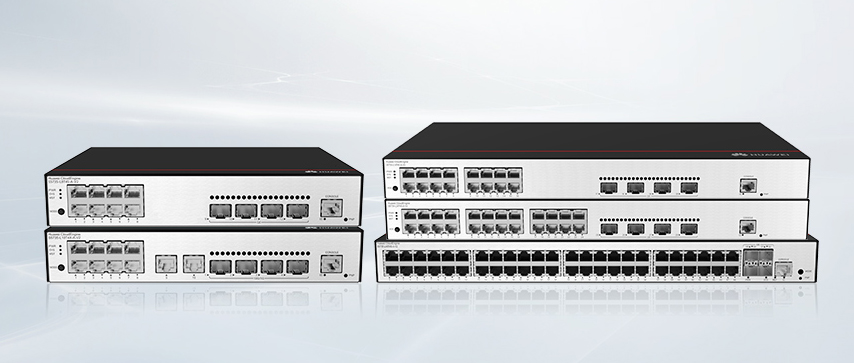

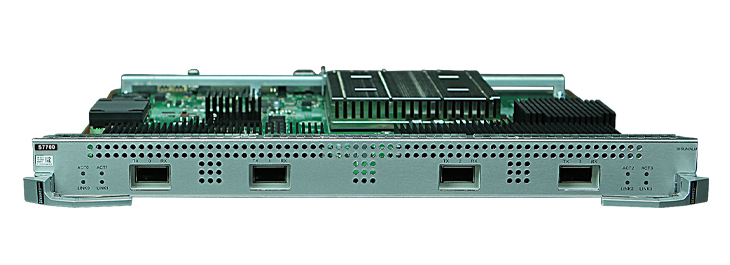

















The U.S. Food and Drug Administration (FDA) published a draft guidance titled Cybersecurity in medical devices: Quality system considerations and content of premarket submissions. The final version of the guide will: (1) Offer recommendations to the industry on cybersecurity considerations for devices, (2) Help the industry identify issues related to Cybersecurity in the design and development of their medical devices, and (3) Provide recommendations for documentation in device premarket submissions. The deadline for public comments is July 7, 2022.
 Tags quentes :
Desenvolvimento de capacidades
Desenvolvimento
Internet das Coisas
Saúde digital: aplicações tecnológicas
Tags quentes :
Desenvolvimento de capacidades
Desenvolvimento
Internet das Coisas
Saúde digital: aplicações tecnológicas
Cadastre-se agora para Ações Semanais de Promoção
100% free, Unsubscribe any time!
Add 1: Room 605 6/F FA YUEN Commercial Building, 75-77 FA YUEN Street, Mongkok KL, HongKong Add 2: Room 405, Building E, MeiDu Building, Gong Shu District, Hangzhou City, Zhejiang Province, China
Whatsapp/Tel: +8618057156223 Tel: 0086 571 86729517 Tel em HK: 00852 66181601
Email: [email protected]
 English
English Pусский
Pусский Français
Français Español
Español Português
Português